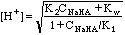Type
pH = -log [H+]
[H+] = Kw/[OH-]
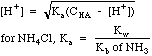
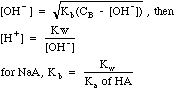
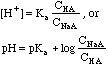
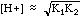
Type
|
Examples
|
Approximate
Formulas
|
| Strong
Acid
|
HCl,
HNO3, H2SO4 (first proton), HClO4
|
[H+]
= CSA, (if CSA > 10-6)
pH = -log [H+]
|
| Strong
Base
|
NaOH,
KOH, Ba(OH)2
|
[OH-]
= CSB, (if CSB > 10-6)
[H+] = Kw/[OH-]
|
| Weak
Acid
|
Acids
with a Ka, e.g. CH3COOH, or salts of weak bases, e.g.
NH4Cl.
|
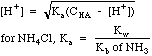
|
| Weak
Base
|
Bases
with a Kb (mostly amines, e.g. NH3), or salts of weak
acids, e.g. CH3COO-Na+.
|
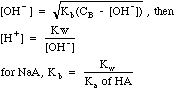
|
| Buffer
|
Mixtures
of weak acids and their conjugate bases, e.g. CH3COOH mixed with
CH3COO-Na+
|
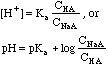
|
| Amphiprotic
(polyprotic)
|
NaHCO3,
KHPhthalate
|
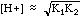
|
Some Extensions to the formulas above:
The quadratic equation may need to be solved. For ax2+bx+c =
0,
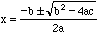
For very dilute strong acids or bases, e.g. CHCl = 10-8 M HCl, charge balance gives us
[H+] = [Cl-] + [OH-], from which we get
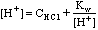 .
Rearrange into standard quadratic equation form and solve for
[H+].
.
Rearrange into standard quadratic equation form and solve for
[H+].
For weak acids or bases, if successive approximation does not converge, convert the equation into the quadratic equation form and solve for [H+] or [OH-]
Dilute Buffers may require this extended equation:
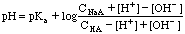
The Full Amphiprotic Equation is: