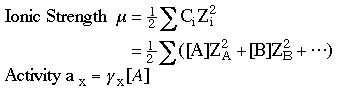
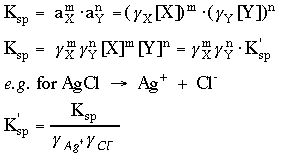
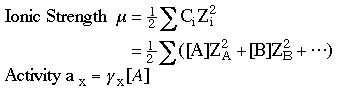
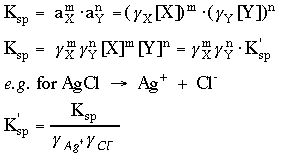

Remember--to include the effect of ionic strength in Ksp problems:
1. Write out the balanced chemical reactions
2. Write out any relevant equilibrium equations, such as Ksp.
3. Set up an Initial/Final Spreadsheet. Put in the millimoles
for all species and record the final volume in milliliters.
4. Carry out all reactions and compute the final millimoles of
all species.
5. Using the millimoles and the total volume, compute the concentrations
of all major ions.
6. Compute the ionic strength.
7. Compute or look up the activity coefficients and calculate
Ksp', the effective Ksp.
8. Solve the equilibrium problem using Ksp' in place of Ksp.