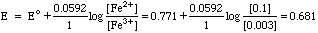
Spectro and Redox
Thursday 21 November, 1996 Name ___Answer Key________
Here is a portion of the Redox table we have been using:
Cr2O72- + 14H+ + 6e-
|
=
|
2Cr3+
+ 7H2O
|
+1.33
|
| O2
+ 4H+ + 4e-
|
=
|
2H2O
|
+1.229
|
| IO3-
+ 6H+ + 5e-
|
=
|
1/2
I2 + 3H2O
|
+1.195
|
| Cu2+
+I- + e-
|
=
|
CuI
|
+0.86
|
| Ag+
+ e-
|
=
|
Ag
|
+0.799
|
| Fe3+
+ e-
|
=
|
Fe2+
|
+0.771
|
1. Write the balanced complete reaction between 02 and Fe2+ using half reactions from the table above.
O2 + 4H+ + 4e- = 2H2O4Fe2+ = 4Fe3+ +4 e-
-----------------------------
O2 + 4H+ + 4Fe2+ = 4Fe3+ + 2H2O
2. Write the balanced half reaction for O2 going to H2O2.
O2 + 2H+ + 2e- = H2O2
3. For the following electrochemical cell: (left and right have been identified for the orientationally challenged.)
A. Use the Nernst Equation to calculate the potential of the Left side.
B. Use the Nernst Equation to calculate the potential of the Right side.
C. Which side is the Anode?
The Anode is the side in which oxidation takes place in the solution. The left side is the more negative (or less positive, if you prefer), so the electrons must come from there. For electrons to come from there, they must be extracted from the solution. The solution loses electrons: that's oxidation, so the left side is the Anode.
D. If we connected a voltmeter to this cell, which side should the Red (+) lead and which side should the Black (-) lead go to have the meter read in the right direction?
The right side is the more positive, so the Red (+) lead must be attached there. The left side is attached to the Black (-) lead, since it is the least positive (or more negative, if you prefer).
E. If we connected a Volkswagen tail lamp to this cell, which way would the electrons flow?
The right side is more positive, so the electrons will flow toward it.
F. With the electrons flowing here as a galvanic cell, at which electrode would reduction take place? Would this change if this were an electrolytic cell?
In the Galvanic cell shown above, the electrons travel toward the positive (right) side. Reduction takes place in the solution on the right side, since the species in solution would gain these electrons. Reduction is a gain of electrons.
In this electrolytic cell, a "battery charger" actively forces electrons down the throat of the negative side. This direction of current flow is the reverse of that found when the cell is used as a source of current, as when it was attached to the lamp. So, although the left side is still negative and the right side is still positive, reduction is now taking place on the left side in this electrolytic cell. Note that when you jump start a car, always hook positive to positive and negative to negative, as was done above.
4. 20.0 mL of 0.0100 M Cr2O72-, 3.00 mL of 18 M H2SO4 and 30.0 mL of 0.100 M Fe2+ are mixed together. Compute the concentrations of all species after reaction, and compute the potential of a Pt electrode placed in this mixture vs. a Ag/AgCl reference electrode (0.228 V in 1 M KCl).
Balanced Reaction: __Cr2O72- + 14H+ + 6Fe2+ = 2Cr3+ + 6Fe3+ + 7H2O__
Cr2O72-
Cr3+ H+ Fe2+ Fe3+ Volume Initial millimoles
0.200
0
108
3.00
0
20+3+30
Final millimoles
0
0.400
108-2.8= 105.2
3.00-1.20 = 1.80
1.20
53 mL
Final Molarity 0.40/53=
0.00755M
105.2/53= 1.98M
1.80/53= 0.0340M
1.20/53= 0.0226
= 1.20 millimoles of Fe2+ used.
0.200 millimoles Cr2O72-
6 Fe2+ 1 Cr2O72-
=2.8 millimoles of H+ used.
0.200 millimoles Cr2O72-
14 H+ 1 Cr2O72-
Emeas = Etest - Eref = 0.761 - 0.228 = 0.533 volts
5. 0.4532 grams of an iron ore unknown were dissolved and made up to volume in a 500 mL volumetric flask ("Unknown Stock"). 50.0 mL of the Unknown Stock were made up to volume in a 250 mL volumetric flask ("Unknown Intermediate"). 14.5 mL of the Unknown Intermediate were placed in a 100 mL volumetric flask ("Final Flask"), color forming reagents were added, and the concentration of Fe in the Final Flask was determined to be 1.65 micrograms/mL. Compute the %Fe in the iron ore.
1.65 micrograms
100 mL 250 mL 500 mL g mL 14.5 mL 50 mL 106 micrograms =0.02845 g Fe
%Fe = (g Fe/g unk)x100% = (0.02845 g Fe/0.4532 g unk) x 100% = 6.28% Fe