The Modular Zoo
(c) David L. Zellmer, Ph.D.
Department of Chemistry
California State University, Fresno
September 6, 1998
Just as animals have many common features, but each performs unique tasks, so too do instruments. Troubleshooting, or just getting the instrument to do what you want, usually involves understanding how each portion of the instrument affects the output. Once the critical module is identified, the analyst can begin to exercise his or her skills in plumbing, optics, electronics, and computer software. Chemistry comes in handy too.
Chemistry 106 students are reminded that as you learn the operation of each instrument used in lab, be prepared to sketch out a more specific block diagram showing how your instrument works; don't just copy one of the general diagrams shown below for use in your lab reports.

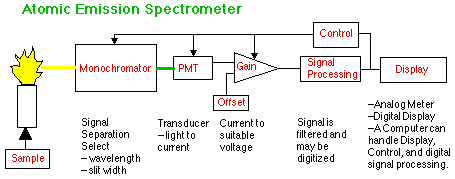
Using the Atomic Emission Spectrometer (AES) as our first example, we first encounter plumbing, as the sample is aspirated or pumped into the flame or plasma torch. The details of sample introduction can be quite complex, but if you see no signal change at the other end when you change sample concentration, you may have a plumbing problem and will have to learn how it works. Playing with the computer will not help a clogged inlet port or a faulty gas valve.
The monochromator is an optical device that allows you to separate the analyte signal from the signal due to the sample and torch matrix. Understanding the properties of spectra will be essential to proper choice of wavelength and slit width.
Once the proper signal exits the monochromator it must pass through a transducer that will convert light to electric current. The photomultiplier tube (PMT) is one such device, although many others are used as well. Control of the voltage to the PMT is required to produce a readable signal with a minimum of noise.
The current from the PMT is next amplified and offset. Offset means setting the blank signal from the PMT to somewhere near the zero point (also called the baseline) of the next module. Gain, or amplification, is needed to make the size of the signal match the input requirements of the next module. Attenuation is the opposite of Gain. If you set the Attenuation of your GLC integrator to 512, the signal will be 1/512 of its original size. "Sensitivity" is yet another name for Gain. A Sensitivity of 0.01 Absorbance units probably means that the output signal will be 10 times bigger than a sensitivity setting of 0.10 Absorbance units.
Signal processing usually involves analog filtering to reduce the noise. The simplest of these is the RC filter.
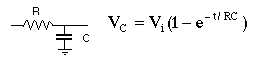
The value of RC is sometimes called the "time constant" or "rise time." When Vi = 1 volt is applied to the circuit it will take RC seconds for Vc to "rise" to 1-1/e volts. There may be controls with these labels on them on your instrument. The effect is to reduce the size of high frequency noise, but too large a time constant can distort a rapidly changing signal.
Another common type of signal processing is Analog to Digital Conversion (A/D), which generates the digital numbers that a computer can understand.
Computers are the ultimate display device, since they can convert the numbers to any form you wish. Simpler displays are digital and analog (meter) readouts.
All instruments have some sort of control circuitry. Advanced instruments turn this control over to the computer that also generates the display. Getting the computer to do what you want can sometimes be more difficult than turning the knob yourself. On the other hand, if the instrument is set up just the way you want it, and this state of control is stored in the computer, you can restore this state later on with a single command.
Look for these same features on the instruments which follow.
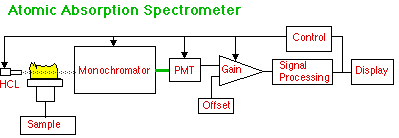
The big change from AES is the use of a Hollow Cathode Lamp (HCL) to put wavelengths specific to the analyte through a long, thin flame. Absorption of this HCL radiation, rather than emission from the flame, is the measure of how much analyte is present. More advanced instruments may have additional optical components to provide Double Beam mode and Background Corrector mode.
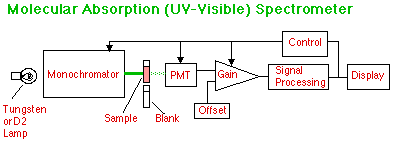
In this single-beam UV-Visible spectrometer, the light from a tungsten source for the visible, or a deuterium source for the UV, or other source of continuous radiation is first separated and a wavelength selected to pass through the sample which is held in a sample cuvet. The light passing through this sample is divided by the light passing through a blank to compute Transmittance.
In a double beam instrument the light passes through both cells using additional optics. The control and signal processing automatically computes transmittance or absorbance. The control can also scan a wavelength range and compute a spectrum which can then be displayed.
The PMT can be replaced with an array of photodiodes and the optical path altered to simultaneously read all wavelengths in the UV-Visible. A computer reads these wavelengths and stores them in an array. From the sample array and the blank array an entire spectrum can be computed and displayed without the time required for scanning the monochromator.
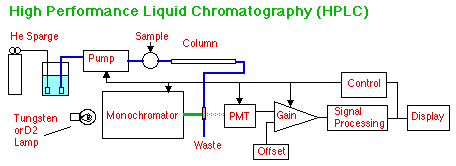
HPLC pumps a liquid mobile phase through a column to separate the components of the analyte which partition between the mobile and stationary phases in the column. A UV-Visible detector is often used to quantify the peaks of separated analyte that come off the column. Computerized instruments can control the He sparging and the sample introduction as well. Other types of detectors are possible with HPLC, include Mass Spectrometry and Electrochemical detectors. See these below. Note too, that we could have simplified this block diagram by simply directing the column output to a box labeled "UV-Visible Detector," but the new flow-through cell would not have been shown.
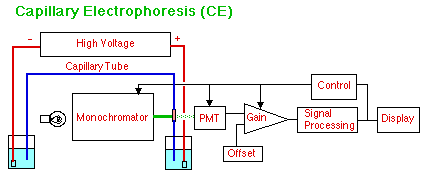
In Capillary Electrophoresis (CE) the analyte components move according to their mobility as ions in an electric field. No pump is used to move the mobile phase, although electroosmotic pressure does cause the buffer solution to move slowly through the capillary tube making Capillary Electrochromatography (CEC) possible as well using micells as a second phase. The sample is introduced by placing the front end of the capillary in an unknown solution and giving it a short burst of high voltage or just holding it up for a brief gravity feed. Detection can be by UV-Visible, as shown, with a small bare spot scraped in the covering of the hair-like capillary tube for a rather tricky short path-length UV-Visible "cell." Electrochemical and mass spectral detection are also used.
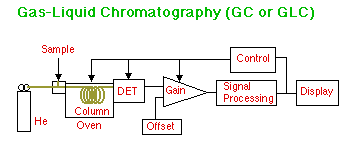
In Gas-Liquid Chromatography (GLC), a sample is injected through a septum into a heated injector block that turns it into a gas. This plug of gas is swept through the column by the helium mobile phase where the analyte partitions between the helium gas and the liquid phase coating the particles inside the heated column. Various types of detectors are used, including Thermal Conductivity (TCD) (in which case dual columns are used), Flame Ionization (FID), Electron Capture (ECD), and Mass Spectrometry (GC/MS). The computer controls not only the detector electronics, but the column, detector, and injector temperatures as well. More advanced instruments add computer controlled sample injection and gas flow rate. Columns can be either Packed or Capillary. Capillary columns have the stationary phase coated on the inside walls of the tube and give much higher resolution than do columns filled with packed particles.
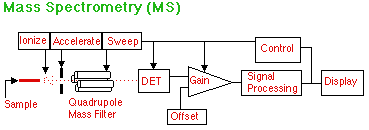
The output from a capillary gas chromatograph has the sample in convenient form for mass spectrometry. Electron or ion bombardment breaks up the molecules of analyte and puts a charge on them. They are then accelerated toward a quadrupole mass filter which only allows fragments of a certain z/m (charge to mass) ratio to pass through to the detector. Since the control sweeps the values of z/m allowed, a complete mass spectrum is acquired in a few seconds or less. This is fast enough to get an identification of every peak that comes out of the GC. Other designs of mass spectrographs are also available such as Time of Flight and Magnetic Sector instruments. Other sources for analyte input are Plasma Torches, HPLC, CE, and Matrix Assisted Laser Desorption Ionization (MALDI). Liquid output from HPLC and CE can be converted to a fine mist with high voltage electrospray or vaporized with applied heat. MALDI can be used on solids and macromolecules.
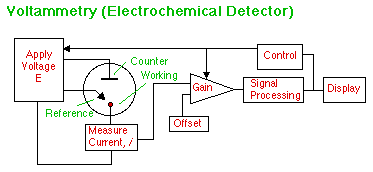
We remember from general chemistry that substances are oxidized or reduced at particular potentials. Tables of these potentials were found next to their half reactions at the back of the book. These potentials correspond to energy levels in solution that can be investigated by applying a voltage to a Working electrode and observing if any chemical reaction takes place that results in a transfer of electrons (an electrical current). The potential of the the Working electrode is monitored vs. a stable Reference electrode in solution. The current needed to maintain this potential is supplied via the Counter electrode. By controlling the rate of change of potential with time, "spectra" of the electrochemical potentials at the electrode surface can be obtained. By holding the working electrode at a potential where a particular analyte will produce a current, we can have an Electrochemical Detector for HPLC or CE.
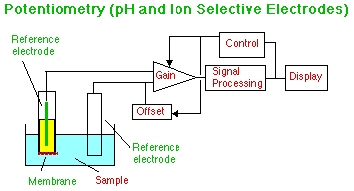
The familiar pH meter and glass electrode work by measuring the potential across a membrane caused by the adsorption of hydrogen ions from the sample. Proper choice of Gain and Offset will allow the display to show the correct pH directly. By changing the membrane and the inner filling solution other ions can be sensed. More complex membranes can measure the flow of oxygen or ammonia across the membrane. (Oxygen detection may be by potential, or by using the Voltammetry apparatus shown above.) By binding enzymes to the membrane, electrodes can be made to respond to specific types of molecules. These electrodes are sometimes called Biochemical Sensors.
Many other instruments are in common use in laboratories, but their electronics and signal processing are far more complex than can be shown in a simple diagram. Nuclear Magnetic Resonance (NMR) and Fourier Transform (FT) versions of NMR (FT-NMR) or Infrared Spectrometry (FT-IR) are examples that will be covered elsewhere in this course.
For questions or comments, please contact
Dr. David L. Zellmer
Department of Chemistry
California State University, Fresno
david_zellmer@csufresno.edu